The Convergys®
POC RT-PCR COVID-19 Testing Platform
- Point-of-care
- Workplace setting
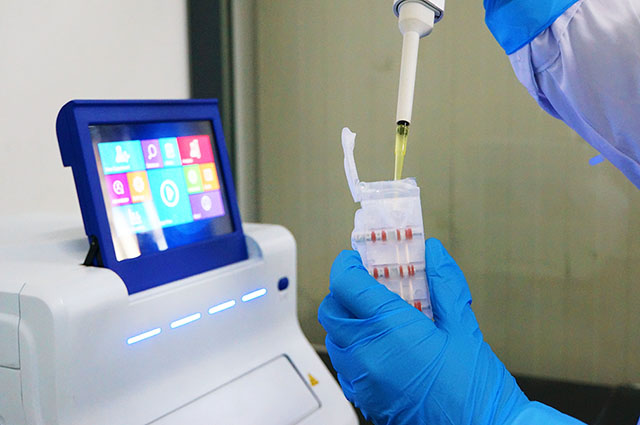
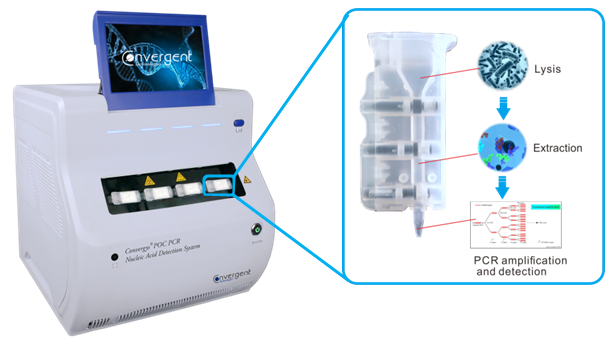
Our Testing Platform integrates WHO endorsed RT-PCR methodology into a convenient and easy-to-use format. The pre-filled, single-use cartridges take care of the complete patient sample processing, including RNA extraction, purification and RT-PCR amplification; all within 90 minutes.
The Convergys® POC RT-PCR COVID-19 Testing Platform directly detects SARS-CoV-2 genetic material.
Features
Qualitative detection of the novel coronavirus SARS-CoV-2
Real-time PCR methodology
Targets specific nucleotide sequences of SARS-CoV-2
Cartridge based kit for the use with the Convergys® POC RT-PCR system
Result within 90 minutes
Internal control material for validation of results
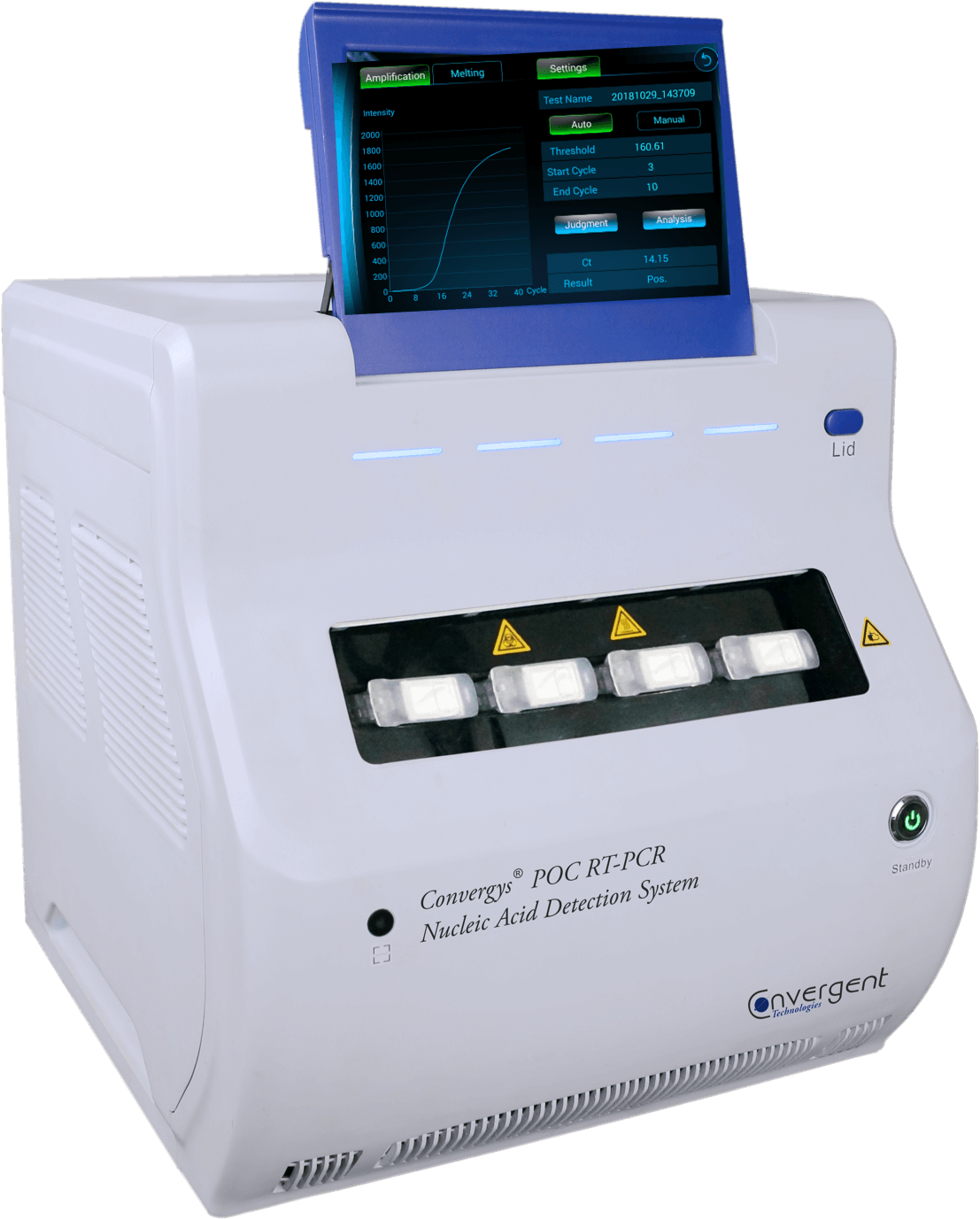
The Convergys® POC RT-PCR system is fully automated:
- Easy operation
- Reduced hands-on time
- Reduced exposure to infectious material
- Reduced risk of application errors
Direct application of sample material, no previous extraction is required